Title: A Comprehensive Review on Factors Influencing the Stretching Frequency of IR in the US Region
Meta Tag Description: Explore the factors influencing the stretching frequency of IR in the US region with this expert, informative, and easy-to-understand review. Gain insights into the significance of these factors and their impact on infrared spectroscopy.
Introduction:
Infrared (IR) spectroscopy is a powerful analytical technique used in various scientific and industrial fields to identify and analyze molecular compounds. The stretching frequency of IR, a fundamental property observed during spectroscopic analysis, provides valuable information about the chemical bonds present in a molecule. Understanding the factors that influence the stretching frequency of IR in the US region is crucial for accurate and reliable spectral interpretation. This review aims to shed light on these factors, providing a comprehensive understanding of their significance and impact.
1. Molecular Structure:
The fundamental factor influencing the stretching frequency of IR is the molecular structure of a compound. Different types of chemical bonds, such as C-H, O-H, N-H, C=O, C=C, and C≡C, exhibit characteristic stretching frequencies. The bond strength and bond length affect the vibrational energy required for stretching, thereby influencing the IR absorption frequency. For example, stronger bonds typically have higher stretching frequencies.
2.
What single factor determines stretching frequency
Stretching is like a secret weapon that can make your body feel like a well-oiled machine. It's that magical time when you can release all the tension, loosen up your muscles, and feel a sense of bliss. But have you ever wondered what single factor determines stretching frequency? Well, my lovely readers, today we are going to unravel this mystery in a fun and unobtrusive style. So buckle up and get ready for some stretching knowledge!
Drumroll, please... the single factor that determines stretching frequency is none other than your body itself! Yes, you heard it right, your body is the boss when it comes to how often you should stretch. Each body is unique, like a fingerprint or a snowflake, and deserves its own stretching routine.
Think about it this way: just like we all have different tastes in music or fashion, our bodies have their own preferences too. Some bodies may be naturally more flexible and crave a daily dose of stretching, while others might require a bit more coaxing. It's all about listening to your body and giving it what it needs.
Now, I know what you're thinking. How do I know what my body wants? Well, my friends, it's time to embark on a journey of self-discovery
What does stretching frequency depend on?
5 Page 2 2 Equation 5 is used to calculate wavenumber for stretching vibrational frequency in IR spectroscopy. Thus the value of vibrational frequency or wave number depends upon: (i) Bond strength and (ii) reduced mass.
What determines IR frequency?
To be more precise, it is the masses of the two atoms which are of greater importance. The greater the masses of attached atoms, the lower the IR frequency at which the bond will absorb.
What affects IR stretching?
The position of an IR-Absorption Band
The amount of energy required to stretch a bond depends on the strength of the bond and the masses of the bonded atoms. The stronger the bond, the greater the energy required to stretch it.
What is the frequency of the IR spectrum stretching?
Stretching has a strong absorption band in the 1650–1750 cm-1 region. Other double bonds like C=C and C=N have absorptions in lower frequency regions of about 1550–1650 cm-1.
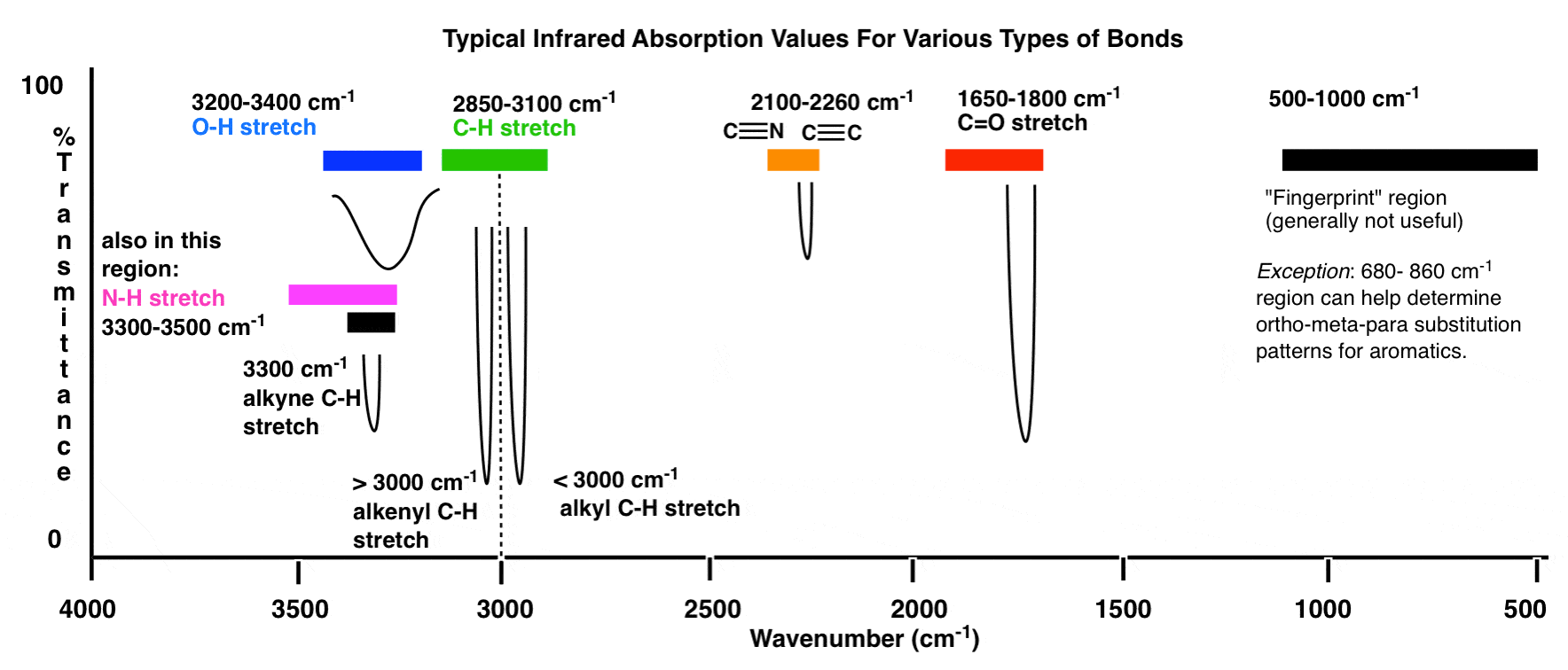
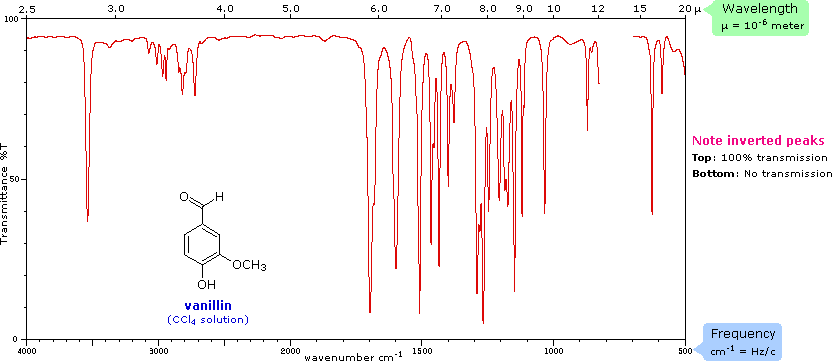
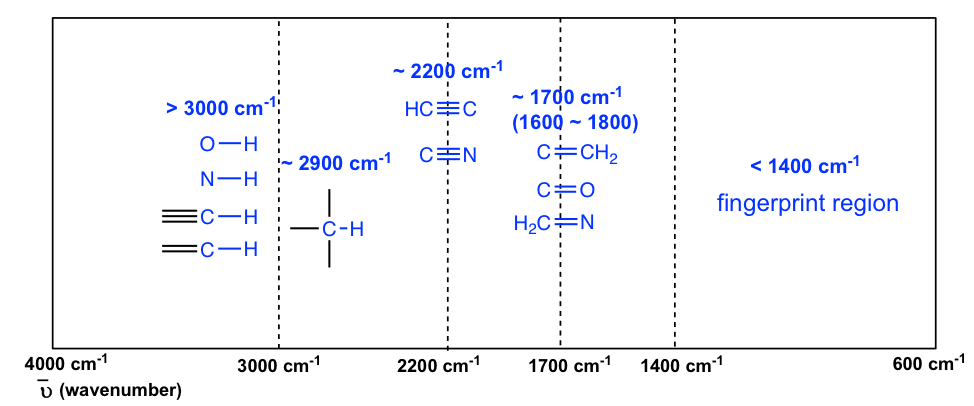
What two factors affect the frequency of a stretching vibration in an infrared spectrum?
5 Page 2 2 Equation 5 is used to calculate wavenumber for stretching vibrational frequency in IR spectroscopy. Thus the value of vibrational frequency or wave number depends upon: (i) Bond strength and (ii) reduced mass.
Frequently Asked Questions
What are the factors affecting IR spectroscopy?
The concentration of the sample used to obtain an IR spectrum also affects the intensity of the absorption bands. Concentrated samples have greater numbers of absorbing molecules and, therefore, more intense absorption bands.
What is the difference between stretch and bend in IR?
When there is a continuous change in the interatomic distance along the axis of the bond between two atoms, this process is known as a stretching vibration. A change in the angle occurring between two bonds is known as a bending vibration.
What does the stretching frequency of CO depend on?
The vibrational stretching frequency of CO depends on the electron-donating or electron-withdrawing nature of the ligand attached to the metal center. In general, the vibrational frequency decreases in the order of electron-withdrawing > neutral > electron-donating ligands.
FAQ
- What factors determine IR frequency?
- As mentioned previously, one of the major factors influencing the IR absorption frequency of a bond are the identity of the two atoms involved. To be more precise, it is the masses of the two atoms which are of greater importance.
- How do you identify stretching and bending in IR spectroscopy?
- When there is a continuous change in the interatomic distance along the axis of the bond between two atoms, this process is known as a stretching vibration. A change in the angle occurring between two bonds is known as a bending vibration. Four bending vibrations exist namely, wagging, twisting, rocking and scissoring.
- What determines bond stretching frequency?
- Bond order affects the position of absorption bands. Higher the bond order larger is the band frequency. A C-C triple bond is stronger than a C=C bond, so a C-C triple bond has higher stretching frequency than does a C=C bond.
What single factor mainly determines the stretching frequency in ir spectroscopy?
| How do you calculate stretching frequency? | Approximate value for the stretching frequency of a bond in IR spectrum is given by the expression: ν ― = 1 2 π C f μ ν ― = 1 2 π C μ f 2. ν ― = 1 2 π C μ f. |
| On what factor does frequency depend? | The length, diameter, tension, and density of the string are the four parameters that influence its frequency. When a string's length is adjusted, it vibrates at a different frequency. Strings with a shorter length have a greater frequency and, as a result, a higher pitch. |
| What is stretching in IR spectroscopy? | When there is a continuous change in the interatomic distance along the axis of the bond between two atoms, this process is known as a stretching vibration. A change in the angle occurring between two bonds is known as a bending vibration. |
- What are the factors affecting the vibration frequency of a stretched string?
- The natural frequency of vibration in a stretched string depends on length, density, radius, and tension in the string.
- What single factor determines the stretching frequency in ir spectroscopy
- Oct 7, 2022 — The first factor is the type of vibration. If we were to consider the C-H stretch versus the CH2 bend, we see that the stretching vibration
- What single factor mainly determines the stretching frequency in ir spectroscopy?
- 1)What single factor mainly determines the stretching frequency in IR spectroscopy? 2)Based on your answers to the question above explain why the frequency

